WQ Playground
This WQ Playground gives you some hands-on experience with the way aeration affects dissolved CO2, alkalinity, and pH.
If you're not familiar with the visual approach to aquaculture WQ management and training used here, you'll find a short interactive "scrollytelling" intro at the WQ Viz website.
Quick-start
The screencast below shows you what to do:
- Select the Alkalinity Calculation radio button
- Drag the target waypoint right-to-left to simulate aeration
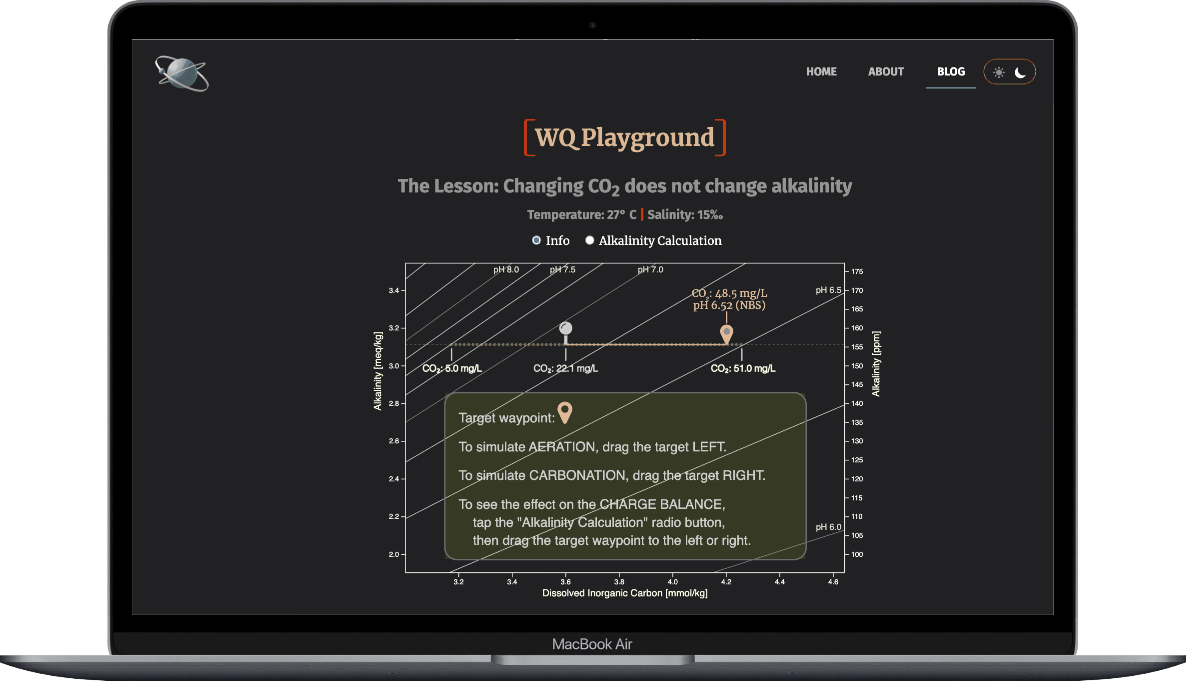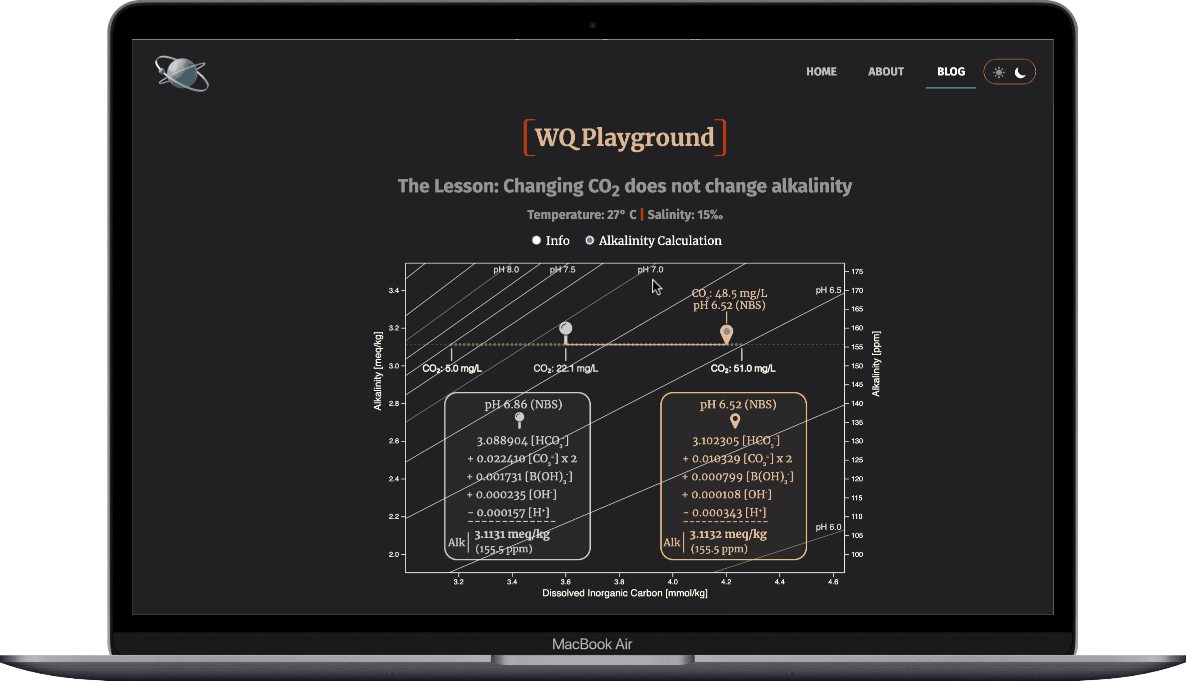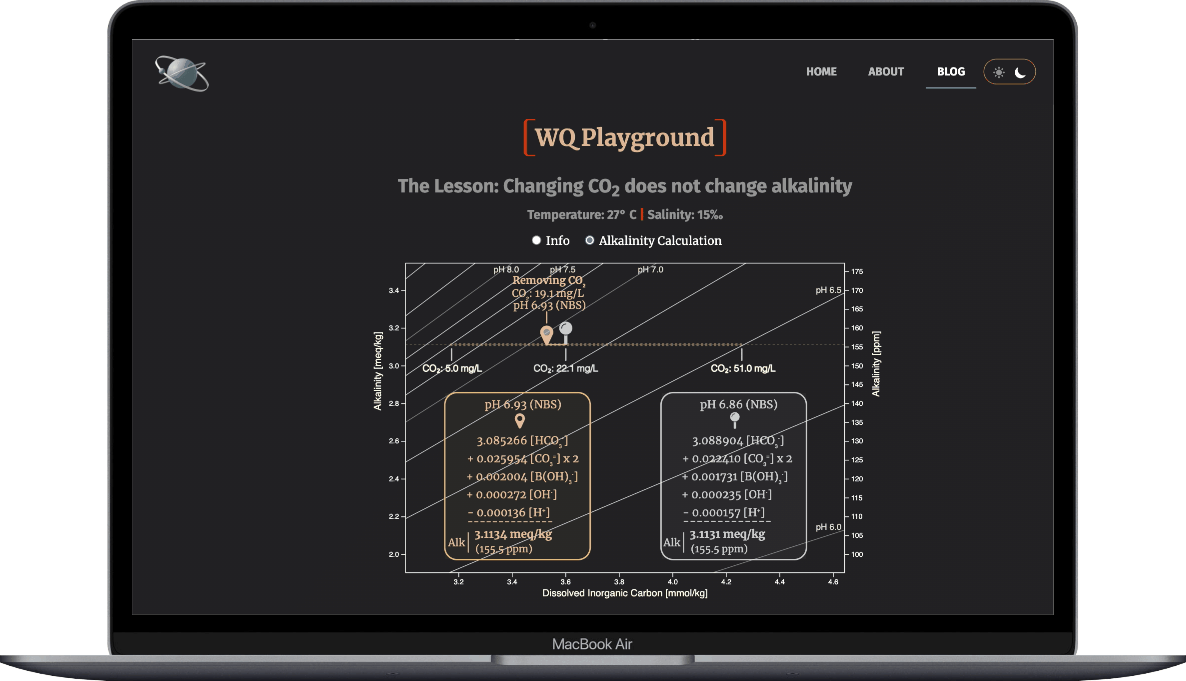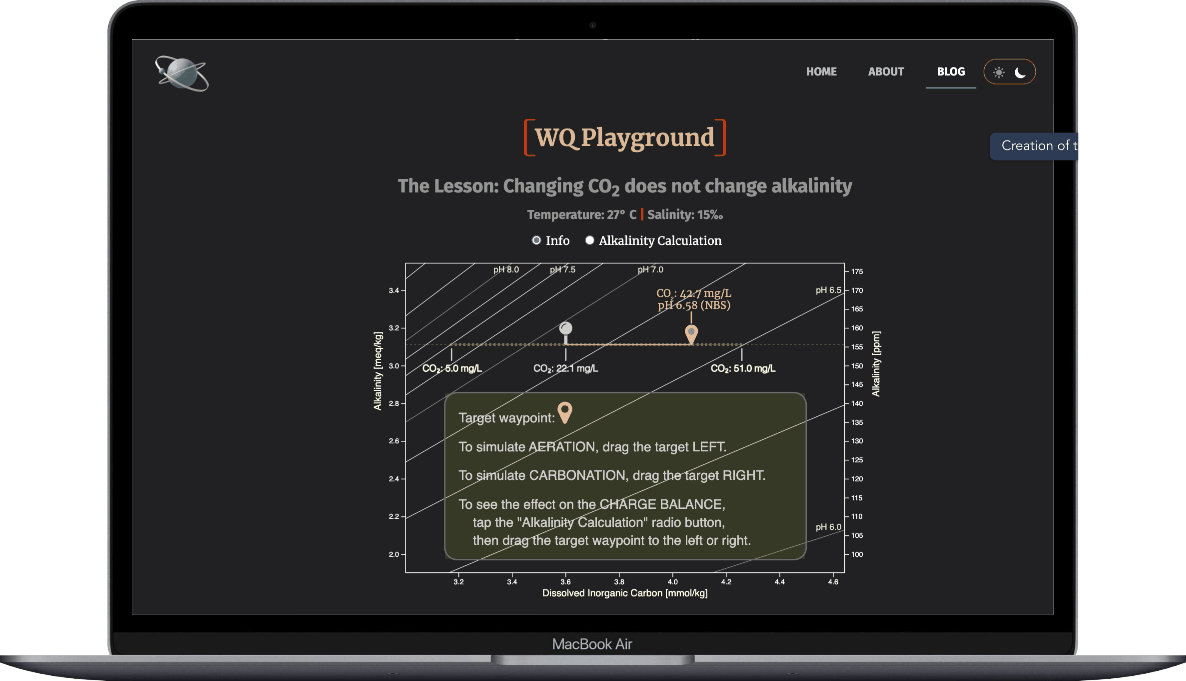
- Observe how the ions that make up alkalinty change
- And note that alkalinity doesn't change
OK. Your turn...
There are two things at play:
- To keep the presentation simple yet still illustrate the basic concepts, we didn't add all of the ions that make minor contributions to alkalinity.
- There's some numerical error owed to how we do the calculations under the hood. It's a trade-off between accuracy and a smoothly functioning user interface.
Here’s one way to wrap your head around what's going on:
When aeration removes CO2 from the culture water...
- HCO3- & H+ concentrations drop
- HCO3- & H+ thus make smaller contributions to alkalinity
- that loss creates a charge deficit among the five TA ions
- the other ions increase enough to cover the charge deficit
Result: The alkalinity doesn't change.
Test your understanding by quizzing yourself.
For example, ask yourself how pH, HCO3-, and CO3= change when L. vannamei respiration adds CO2 to the culture water; then simulate that scenario by dragging in the proper direction to check your answer.
Another useful exercise: Drag the target to any CO2 position, then get out a calculator to add up the charge balance of each ion at that point. Round to two decimal places for the level of accuracy adopted in this post.
That will help convince you that alkalinity doesn't change when you remove CO2 by aerating.