This question arises in aquaculture, aquaponics, aquarium chemistry, & swimming pool management.
The short answer: No, we can't manage alkalinity with aeration.
Table of Contents
Plain Language Summary
You can't manage Total Alkalinity (TA) with aeration.
Aeration lowers carbon dioxide (CO2) in culture water.
That changes the concentrations of the ions that make up alkalinity, such as carbonate and bicarbonate.
But it does that without changing the charge balance among those ions.
That charge balance defines alkalinity.
So, because aeration lowers CO2 without changing that charge balance, aeration doesn't change alkalinity.
That's true when aeration is isolated from other processes, but culture systems host a complex web of interacting parts.
Aeration also raises pH, and pH influences other processes. Some of those increase, and others decrease, alkalinity.
But even taking those pH effects into account, aeration is not a dependable tool for TA control in culture water.
So, in practice, how do you manage TA?
We manage TA by adding precise amounts of reagents that change the charge balance of the TA ions.
The Key Take-away
For practical purposes, our main take-away is this:
Quick Background
Aeration increases dissolved oxygen (O2) and lowers dissolved carbon dioxide (CO2).
Our focus is on CO2 because it reacts with water to produce the two major components of Total Alkalinity (TA), bicarbonate ion (HCO3-) & carbonate ion (CO3=):
Note: Carbonic acid, H2CO3, is in parentheses to indicate that there never is much of it in solution (much less than 1%): It's relatively slow to form, but once formed, it rapidly dissociates to HCO3-.
CO2 also impacts the concentrations of the other ions that make up alkalinity, most notably borate (B(OH)4-).
Those reactions are reversible, so any change in dissolved CO2 (such as from aeration) changes the concentrations of those ions.
That has led some to conclude that, because aeration lowers CO2, it also must lower alkalinity.
Though that might sound reasonable...it’s wrong.
Why? The Charge Balance!
Changes in dissolved CO2 affect the concentrations of all TA ions, but differently and in a way that maintains the charge balance.
For example, as aeration lowers the concentration of CO2, the bicarbonate ion (HCO3-) concentration also decreases, but the carbonate ion (CO3=) concentration increases.
As a result, the single-negative charges lost with the decrease of HCO3- ions are partly replaced by the double-negative charges gained from the increase of CO3= ions.
Carbonate has bicarbonate's back.
As described in the following optional section, the other TA ions pick up the slack so that there is no change in charge balance, which means that there is no change in alkalinity.
[OPTIONAL] A bit more detail...
We can get a fuller picture in the screenshot below, which depicts a slice of the Water Quality Map arbitrarily set at 27° C (80.6° F) and 15‰ (a conductivity of 25.68 mS/cm at 27° C).
Two water-quality waypoints are plotted on the map:
-
marks the water quality before aeration. CO2 is 51 mg/L and pH is 6.50.
-
marks the water quality after aeration. CO2 is 22.1 mg/L and pH is 6.86.
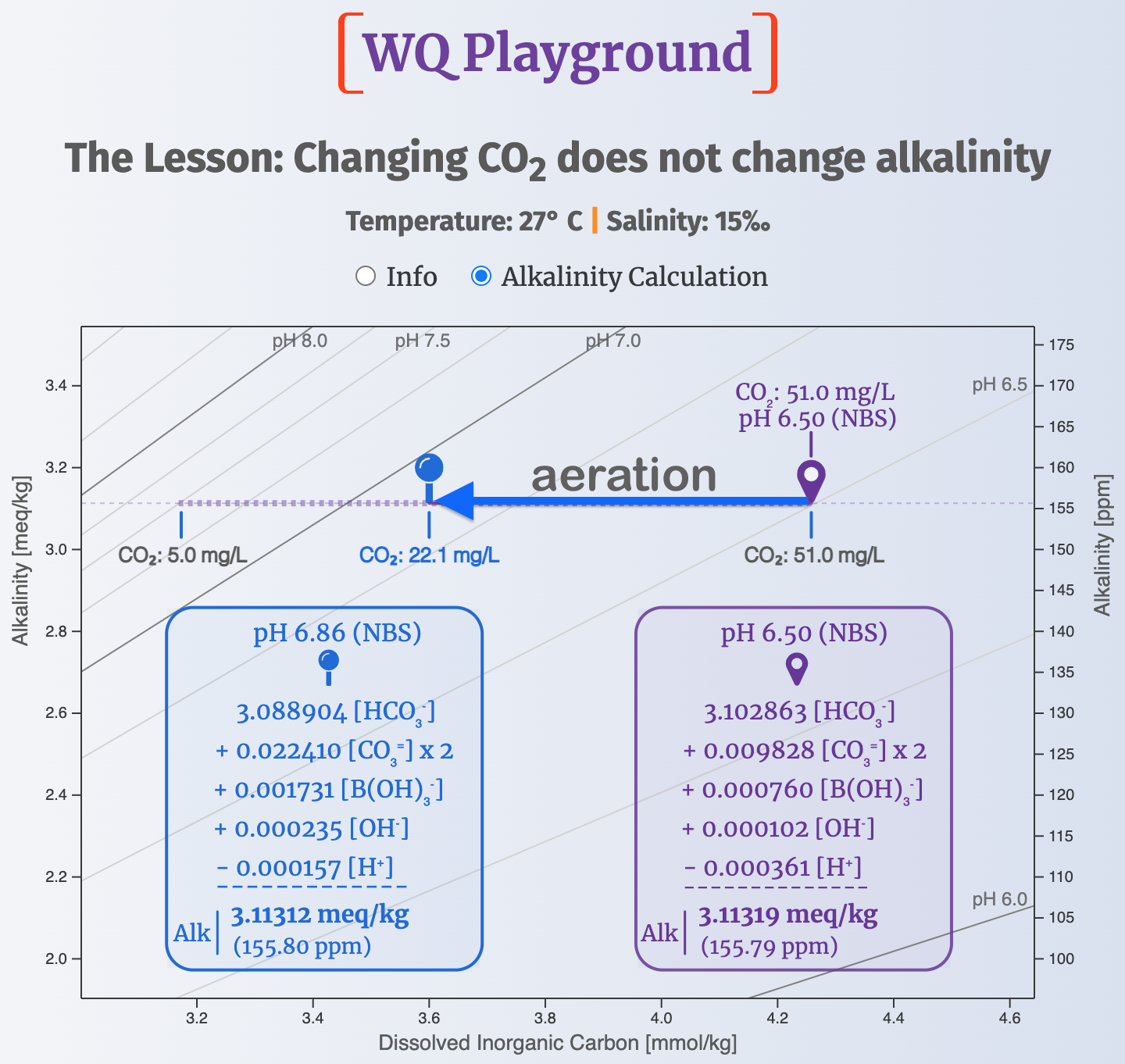
The two boxes — one for each waypoint — list the charge contributions of the five ions that account for the bulk of Total Alkalinity in typical aquaculture systems.
(Don't worry if you're unfamiliar with the units, meq/kg; we'll clear that up in a future post on alkalinity. For now, just focus on comparing the numbers.)
Note how aeration changed the charge contributions of HCO3- & CO3=:
- ⇩ HCO3- charge loss: 3.102863 - 3.088904 = 0.013959 meq/kg
- ⇧ CO3= charge gain: 0.022410 - 0.009828 = 0.012582 meq/kg
So, the CO3= charge gained almost covers the HCO3- charge lost from aerating.
Do the same calculation for each of the TA ions and you'll see that, together, there is no change in charge balance between the two waypoints.
And that means alkalinity stayed the same: ~3.1131 meq/kg (~155.8 ppm)You can play with a live version of this WQ Playgound (link at bottom).
So, stated more concisely...
-
Alkalinity is defined by the charge balance among its ions
-
Changes in dissolved CO2 do not alter that charge balance
-
That means that changes in dissolved CO2 do not alter alkalinity
-
Aeration changes dissolved CO2
That means that aeration doesn't change alkalinity.
That's the correct text-book conclusion when aeration is considered in isolation of all other aquatic processes.
We could end here, but aquaculture systems host a web of many interacting biological, chemical, & physical processes.
We'll gain some useful insight into aquaculture water quality by briefly looking into two related topics:
Other Processes That Change CO2
Aeration isn't the only aquaculture process that changes CO2:


In each case, when CO2 is removed (as in aeration) or added, and isolated from other processes, alkalinity is unchanged.
Other Effects linked to CO2 changes
We've seen that changes in dissolved CO2 themselves do not impact alkalinity.
But aeration and the other processes cited above do more than change CO2; they have other effects which do impact alkalinity.
An algae example
Algal uptake of CO2 is accompanied by uptake of inorganic nitrogen compounds needed to build plant biomass.
The two more important forms of those nitrogen compounds change alkalinity in opposite ways:
-
nitrate (NO3-) uptake increases alkalinity
-
ammonia (NH3) uptake decreases alkalinity
CO2 and pH
pH is sometimes called a "master variable". When it changes, so do many core aquaculture processes.
CO2 and pH are inversely related:
⇩ lower CO2 means ⇧ higher pH
⇧ higher CO2 means ⇩ lower pH
For example:
-
aeration & algal uptake...
- ⇩ lower dissolved CO2
- ⇧ raise pH (only to a certain point when aerating)
-
carbonation & respiration...
[OPTIONAL] A bit about those “certain points”...
Similarly, carbonating can lower pH only to ~pH 5.5; that's the "pH floor". That's too low for most aquaculture, but some hydroponic crops — e.g. cucumbers, basil, sage, Ficus — thrive at ~pH 5.5. (And it's a good pH for espresso.) To push pH lower than that, you'd have to add a mineral acid, such as hydrochloric (muriatic) or sulfuric acid.
(This "ceiling/floor" topic might be expanded in a future blog post.)
Some pH-dependent processes increase alkalinity...
- algal uptake of nitrate
- dissolution of calcium carbonate (CaCO3)
...and others decrease alkalinity...
- nitrification — bacterial production of nitrate from ammonia
- calcification — formation of shrimp carapaces, fish bones...
There are many such pH-dependent processes in culture water, each pulling in a different direction and at a different rate.
What's the net effect of CO2-induced pH changes on alkalinity?
On a small scale — I'm thinking of a low-biomass, non-reef home aquarium — the system might reach a steady-state in which aeration plays its part in the overall alkalinity balance.
But in commercial-scale production, in which biomass is high and changes greatly over a harvest cycle, controlling alkalinity with aeration alone is impractical at best.
Let's re-state the main take-away with a bit of nuance:
Aeration is not an effective tool for controlling alkalinity.
Water Quality Whack-a-Mole
The issue we're facing here looms over most every discussion of aquaculture water quality: The system is highly interconnected, so we have to manage the whole system, not just one part of it.
Otherwise, when we make an adjustment to solve one water-quality problem, others may pop up unexpectedly, and we'll find ourselves playing a game of Water Quality Whack-a-Mole.
So, How do you control alkalinity?
We'll touch on this briefly and expand on it in a separate post.
Let's use some reasoning based on what we learned above:
- managing alkalinity means that we have to change alkalinity
- you know that CO2 doesn't change alkalinity, and you know why: because it doesn't change the charge balance among the TA ions
- so, managing alkalinity boils down to finding ways to change the charge balance among the TA ions
For now, we'll just note that we adjust alkalinity by adding chemical reagents that change the charge balance.
A few examples of common adjustment reagents:
-
increase alkalinity
- sodium bicarbonate (NaHCO3)
- sodium carbonate (Na2CO3)
- calcium hydroxide (CaCO3)
-
decrease alkalinity
- muriatic acid (aka hydrochloric acid of a certain strength)
- sulfuric acid (H2SO4)
How much of each reagent is needed?
Sorting out the consequences of a reagent addition is not a trivial problem, especially as each of those reagents changes pH, too.
Additionally, it depends on the system's water temperature, salinity, current pH, and current alkalinity.
That's a task for the visual approach to water-quality management used in the Water Quality Map.
It's also a topic for a future post.
[Bonus] The connection with the sea

(Image by Dimitris Vetsikas from Pixabay)
Finally, from a broader perspective that pops up regularly in the news, the global rise in atmospheric CO2 is increasing the dissolved CO2 content of the upper mixed layer of the sea.
The effect of added dissolved CO2 on seawater chemistry is the same as described here for aquaculture water chemistry:
pH decreases and alkalinity remains unchanged.
That's an important result in discussions of the sea's role in the global carbon cycle.