WQ Playground
This WQ Playground illustrates how sodium carbonate (soda ash, Na2CO3) increases pH more efficiently than sodium bicarbonate (baking soda, NaHCO3).
Quick-start
The screencast below shows you what to do.
-
Select either checkbox (or both) to display a target icon
- green: sodium carbonate
- blue: sodium bicarbonate
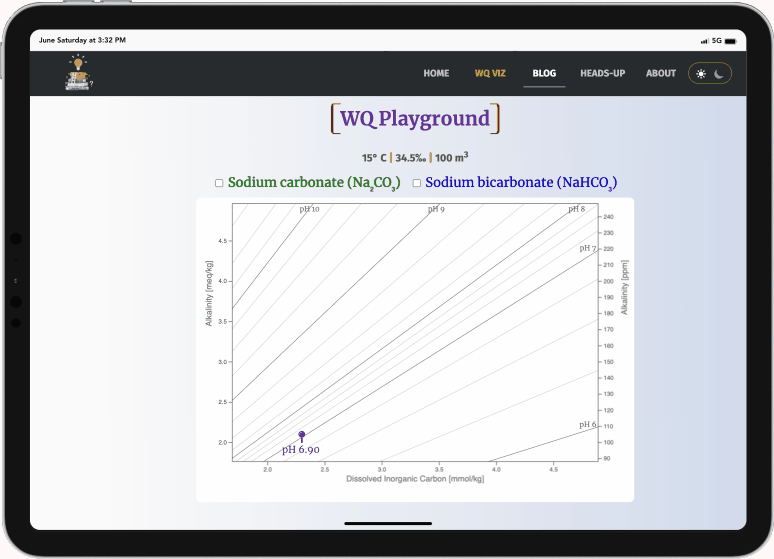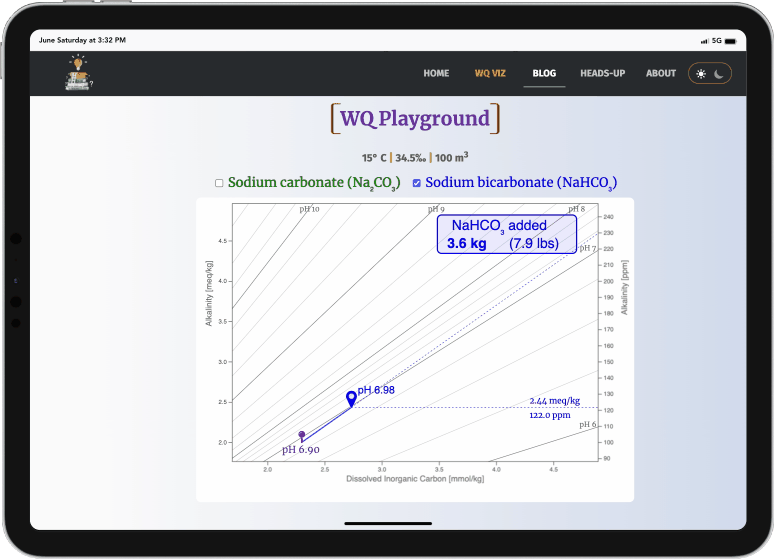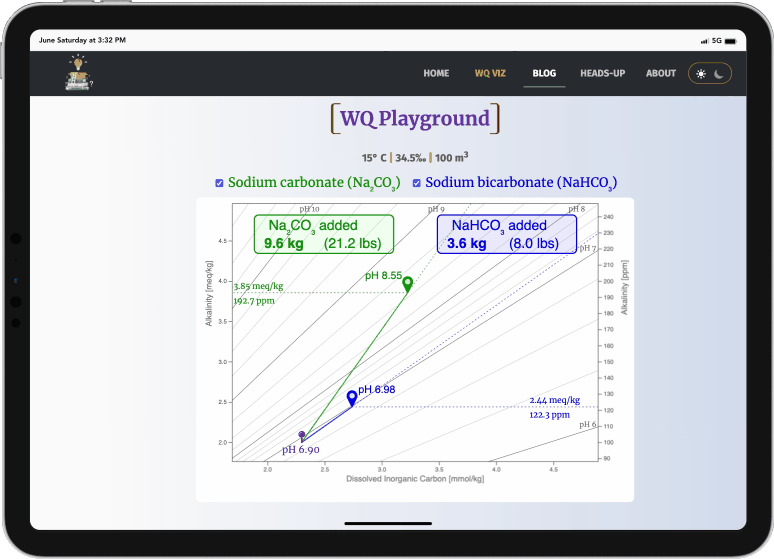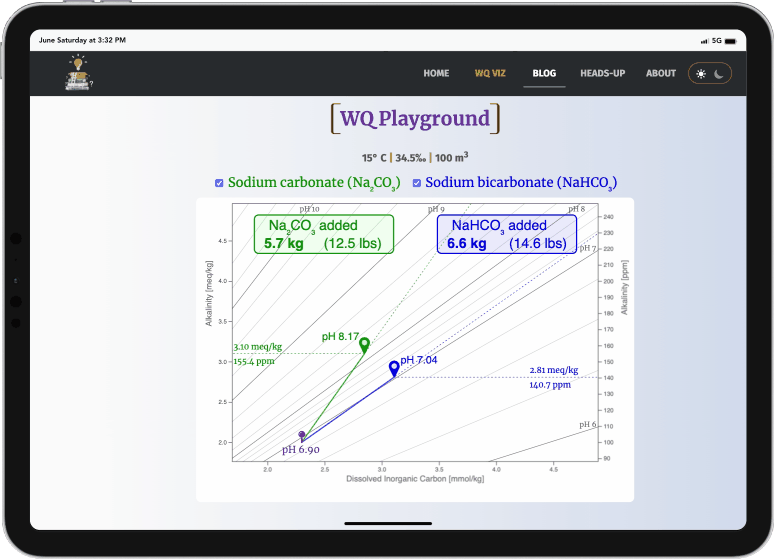
- Drag the green waypoint to simulate adding sodium carbonate
- Drag the blue waypoint to simulate adding sodium bicarbonate
- The amounts added (kg & lbs) and the pH changes are displayed
OK. Your turn...
Suggestions...
- see the effect of adding the same amount of each reagent on pH and alkalinity
- reverse it: How much of each reagent has to be added to get the same pH (or alkalinity)?
Two take-aways...
- sodium carbonate always raises pH much more efficiently than sodium bicarbonate (i.e., it doesn't take as much)
- both always increase alkalinity, but sodium carbonate again does this more efficiently (i.e., it doesn't raise it as high)
Though not illustrated in this playground, the pH increase of both reagents also depends on water temperature, salinity, & the starting alkalinity.
Note that a consequence of sodium bicarbonate's weak effect on pH is that the excessive amounts of bicarb often needed to raise pH will elevate alkalinity beyond the levels found in healthy culture systems.