WQ Playground
This WQ Playground has a software tool that is enhanced to help you get comfortable with converting between moles and mass.
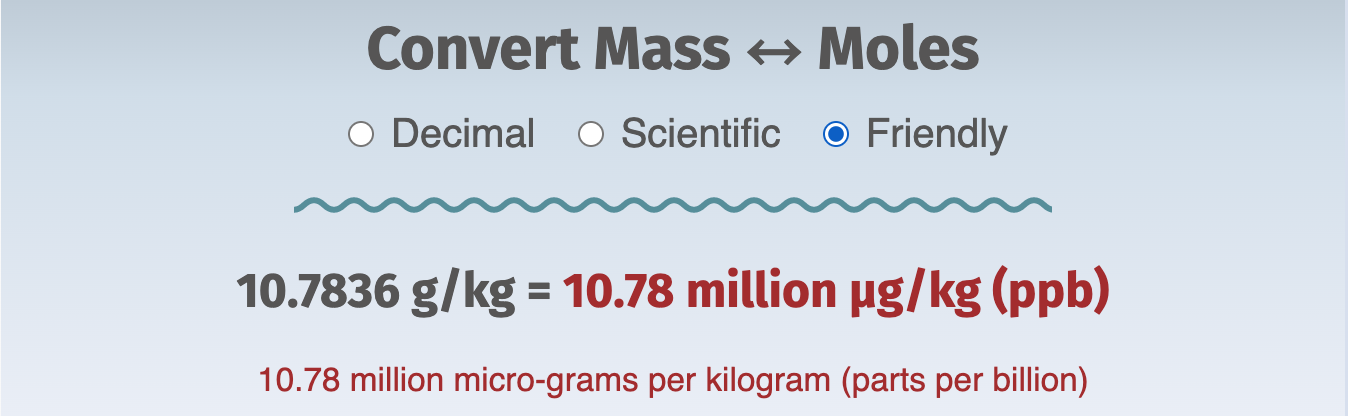
Two of the enhancements are the Scientific and Friendly formats. They're intended to help anyone who might need a bit of practice deciphering scientific notation and reading results, respectively.
Here's an example of the three formats:
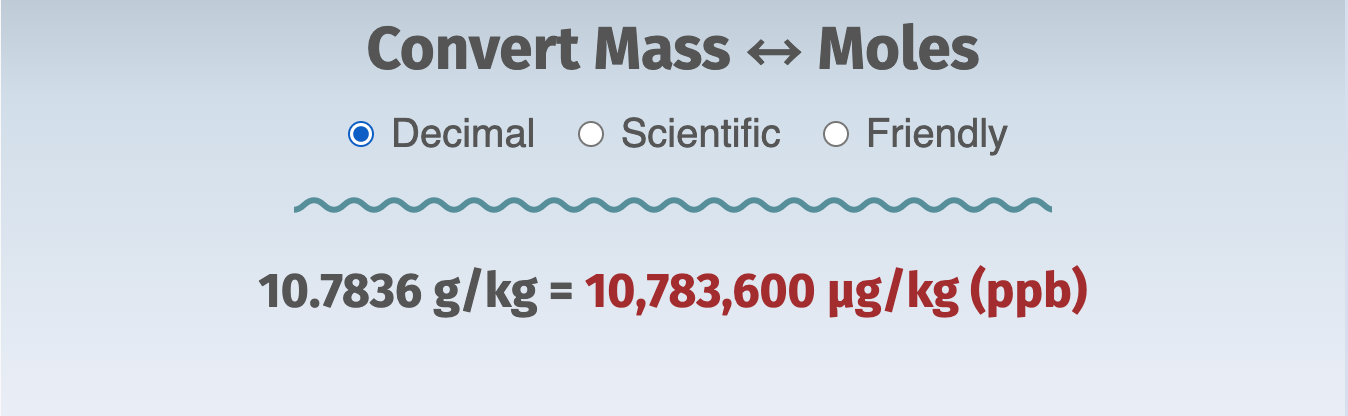
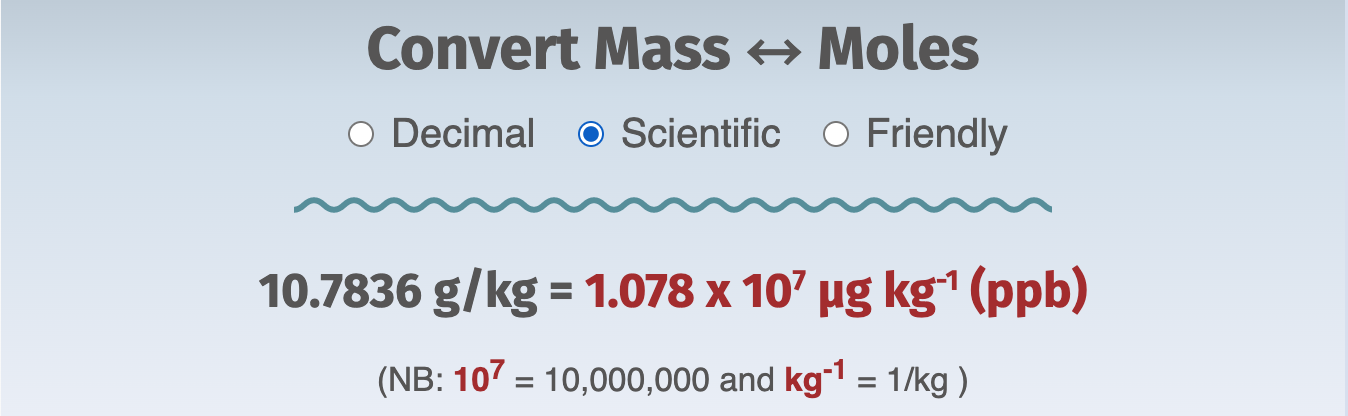
Quick-start
The 20-second QuickTime movie below shows you what to do.
It uses the result from our worked example, 8.215 mg/L CO2, and converts it back to μmol/L.
- Select Carbon dioxide (CO2): 44.0095 g/mol from the "Molar Mass Selection" drop-down menu
- Enter 8.215 mg/L in the Input Data field
- Select μmol/L (the last item) in the Result drop-down menu
- The answer, 186.6642 μmol/L (μM), is displayed
- Tap the Scientific radio button for scientific notation
- Tap the Friendly radio button for the text description
OK. Your turn...
Three example problems
You can get some practice using the converter with these three problems. (If you want, crank them out by hand, and then check your answers with the converter.)
An experiment raised L. vannamei at very low pH to reduce the toxic un-ionized ammonia to which the crop was exposed.
The researchers reported a CO2 concentration of 0.85 mmol/L.
millimoles per liter?! You prefer dealing with CO2 in mg/L.
Q: Convert their 0.85 mmol/L to mg/L.
You may come across research that expresses concentration as per-kg instead of per-L. That simplifies comparisons among water samples taken at different temperatures because mass doesn't change with temperature but volume does.
We mention that here just to prep you for the next two problems.
You're concerned with the sodium-to-potassium ratio (Na:K) of your long-running RAS culture water.
You want it to reflect the ratio in seawater, so you look up the concentration of the sodium ion (Na+) in standard seawater.
It's given as 0.46906 mol/kg. But you want to work with the Na:K ratio in terms of mass, not moles.
Q: Use the converter to change 0.46906 mol/kg Na+ to g/kg.
Finally, let's play with the answer from Problem 2, 10.7836 g/kg, and express it as g/L at two different combinations of temperature and salinity.
We found that the concentration of Na+ in standard seawater (salinity = 35) is 10.7836 g/kg.
You're raising shrimp at 28° and 36‰.
Q1: Convert 10.7836 g/kg Na+ to g/L at 28° C & 36‰.
You're at a meeting and talking with a colleague who raises Arctic char (Salvelinus alpinus) at 7.5° and 22‰.
You both are used to thinking of concentrations in terms of per-L instead of per-kg.
To avoid any confusion, you decide to do a conversion for his culture conditions.
Q2: Convert 10.7836 g/kg Na+ to g/L at 7.5° C & 22‰.
If you were to do that last problem by hand, then you'd have to calculate the water density at each given temperature and salinity. We won't go into that formula here; it's kind of onerous. It's much easier to let the software do the work under the hood.