Many aquaculturists assume that adding sodium bicarbonate always raises pH.
But depending on your culture conditions, adding bicarbonate will either raise pH, lower pH, or — as some have been surprised to find — not change pH at all.
Table of Contents
Plain Language Summary
Sodium bicarbonate (baking soda) does not always raise pH.
Depending on water temperature, salinity, pH, and alkalinity, bicarb either raises, lowers, or doesn't change pH much at all.
For most aquaculture, when pH is...
-
...below ~pH 7, adding bicarb raises pH, but not efficiently
-
...above ~pH 8, adding bicarb lowers pH
-
...within the range of most aquaculture (~pH 7.0 - 7.8), bicarb's effect on pH is so small that some might think their pH meter is broken! (No joke.)
(Note that bicarb always raises alkalinity.)
So, what's going on?
Each unit of bicarb dissolved in culture water adds one unit of alkalinity and one unit of inorganic carbon.
The effect that has on pH is hard to explain with only words and equations.
But this post uses the Water Quality Map to make that easy.
We'll only have to compare the slopes of lines on a map.
So, is bicarb useful for pH control?
Not by itself. Bicarb must be combined with at least one other reagent for precise pH control.
The Key Take-away
Sodium bicarbonate (baking soda) doesn't always raise pH.
Under typical aquaculture conditions, alone, it barely raises pH at all.
Quick Background
pH control is a key part of all water-quality management.
Respiration of aquatic animals, plants, and microorganisms increases dissolved CO2, and higher CO2 means lower pH.
Aeration and certain chemical reagents raise pH.
Of the reagents, many aquaculturists rely on sodium bicarbonate (NaHCO3, baking soda, or just “bicarb”) for pH management.
Their assumption is that adding bicarb always raises pH.
But sodium bicarbonate does not always raise pH.
In fact, more than a few aquaculturists have been surprised when, after adding a massive amount of bicarb, their pH hasn’t changed much at all.
Initially, some suspect that their pH meter is broken.
The issue, however, is not a faulty pH meter: It's the complicated chemistry of the carbonate system.
Instead of plowing through a long technical explanation filled with chemical equations, we'll illustrate bicarb's effect on pH with a few simple visualizations from the Water Quality Map.
[OPTIONAL] Why do many think that bicarb always raises pH?
Visualizing aquaculture water quality
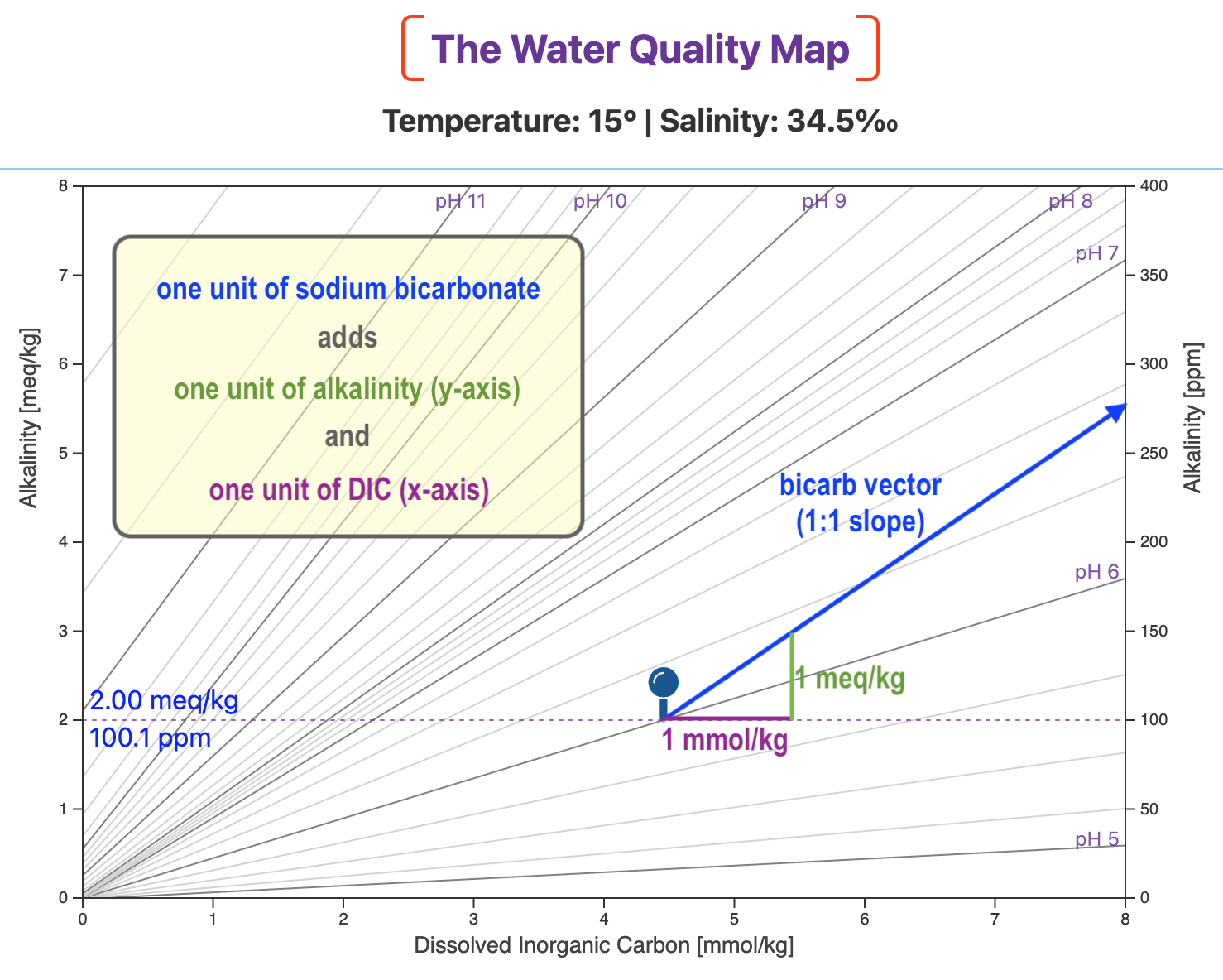
The Water Quality Map is a topographic map of pH, as pictured below for 15° C and 34.5‰.
Oceanographers know this as a Deffeyes Diagram. To serve our purposes, I've adapted it for aquaculture and aquaponics.
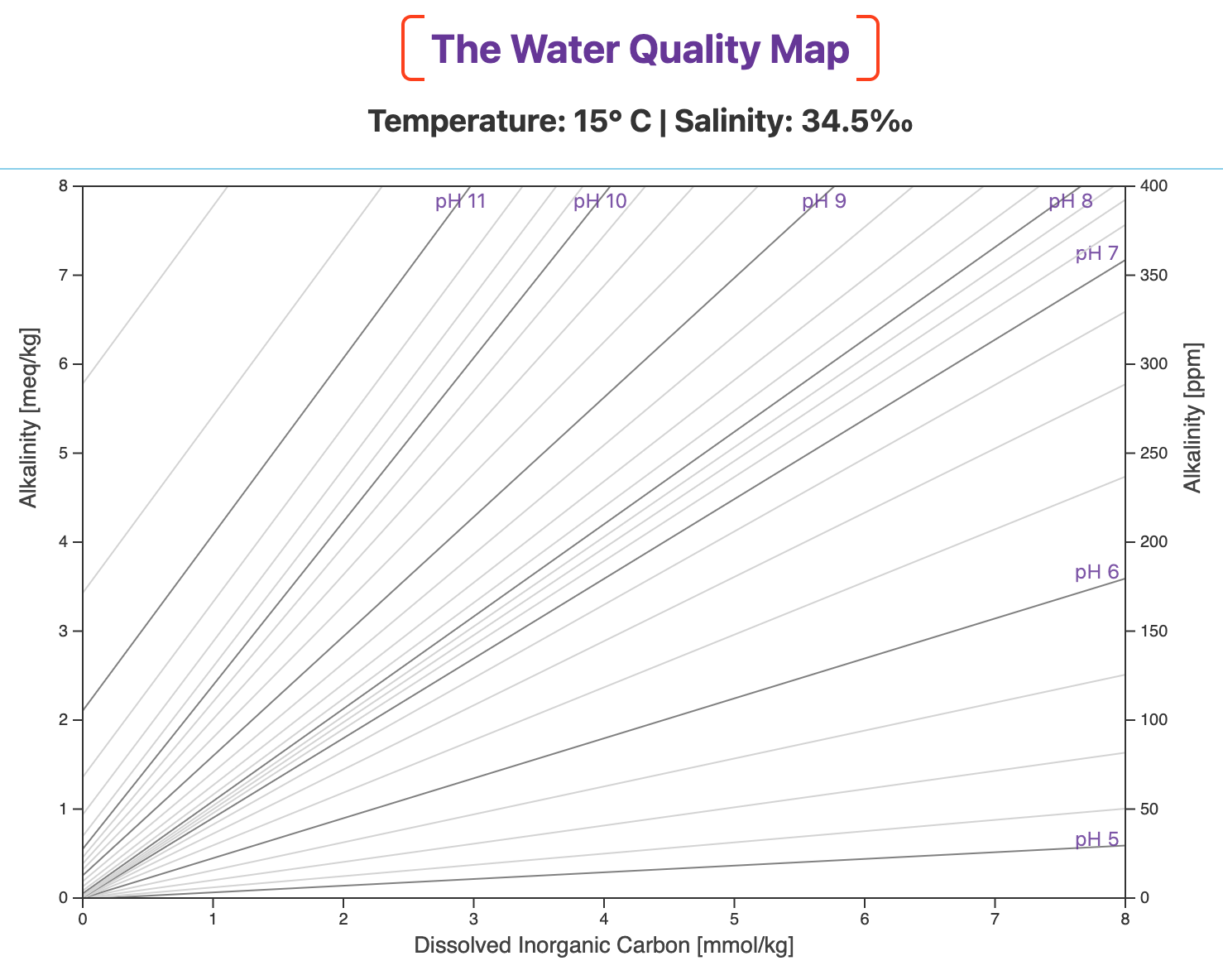
Alkalinity is on the two y-axes and Dissolved Inorganic Carbon (DIC) — the total concentration of bicarbonate ion, carbonate ion, and dissolved carbon dioxide — is on the x-axis.
Lines of equal pH are projected onto the map, just like lines of equal elevation on a topographic map.
Lower pH values have shallower slopes and are found in the lower right of the map.
Higher pH values have steeper slopes and are in the upper left.
The slopes of the pH lines and the relative spacing among them depend on your culture environment's temperature and salinity.
If you're not familiar with this visual approach to aquaculture WQ management, you'll find a short interactive "scrollytelling" introduction at the WQ Viz website.
Our example system:
- 100 m3 (~26,417 gallon US)
- 15° C (59° F)
- 34.5‰ (42.37 mS/cm conductivity)
Before adding any sodium bicarbonate, our water is at pH 6.0 and alkalinity is 2.0 meq/kg (~100 ppm as CaCO3).
The blue pin marks that initial waypoint on the map (below).
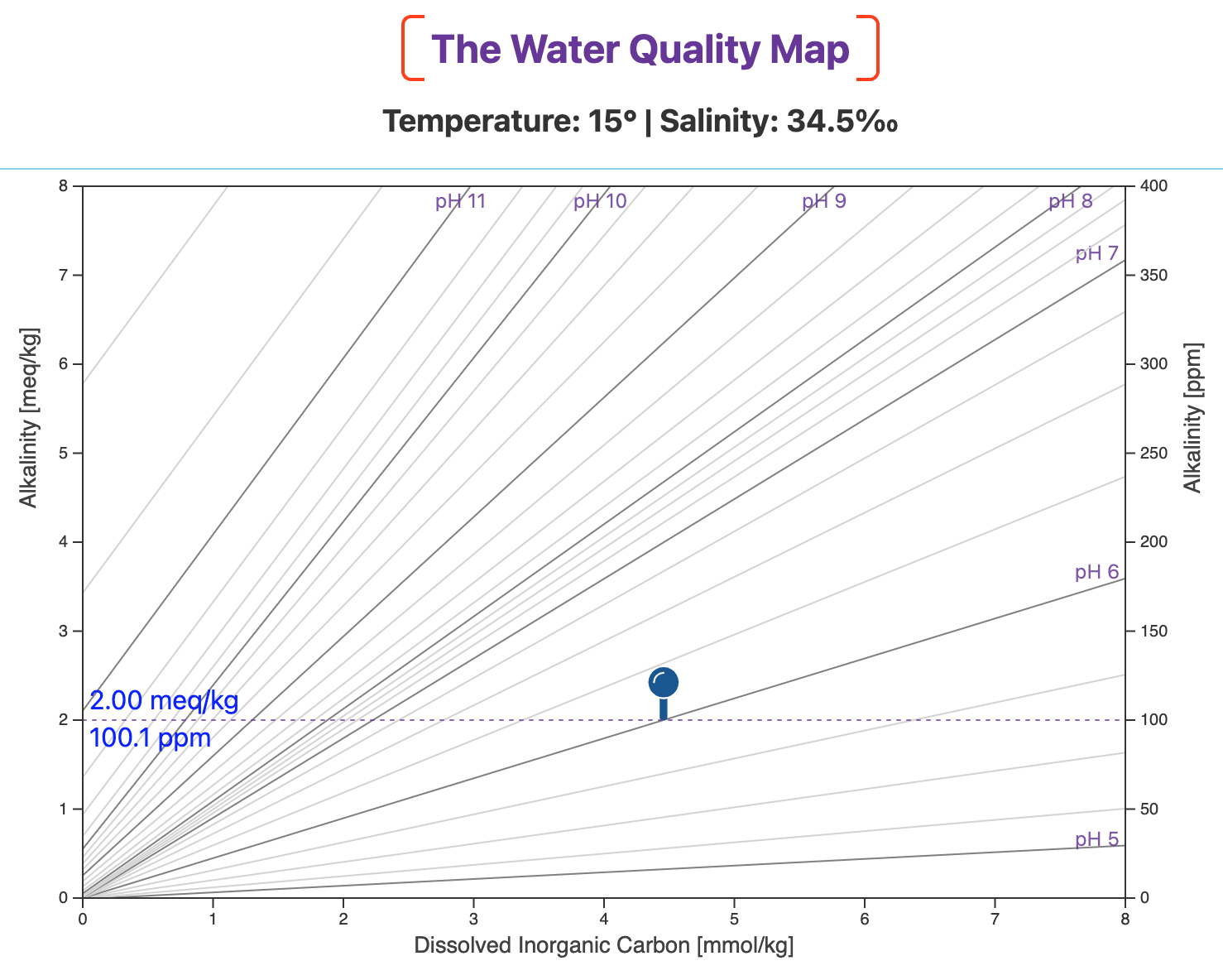
[OPTIONAL] An optical illusion
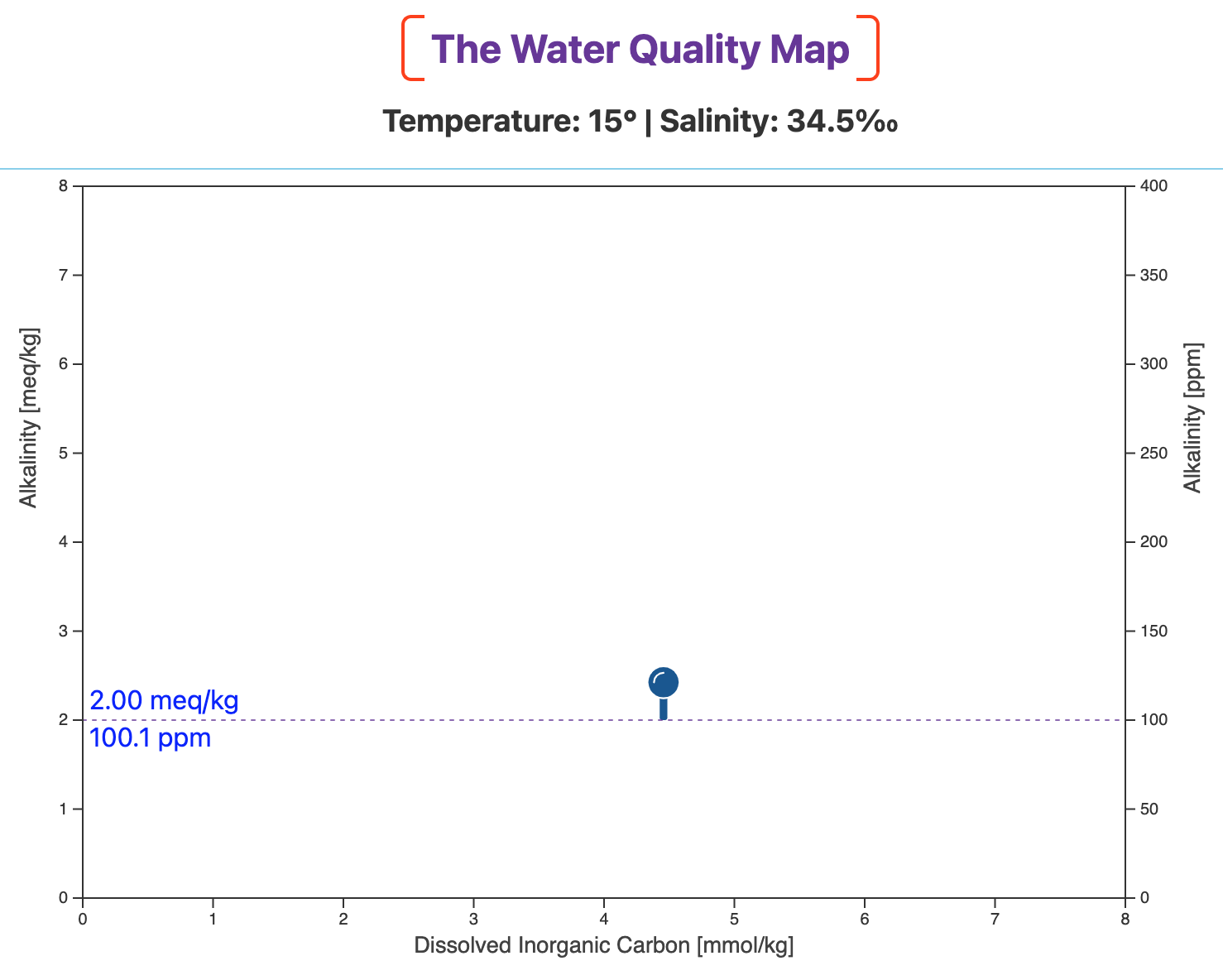
Without the family of pH lines, the dashed line is seen to be horizontal
The Bicarbonate Vector
When you dissolve one unit of bicarb (NaHCO3) in your culture water, it dissociates into a positively-charged sodium ion (Na+) and a negatively-charged bicarbonate ion (HCO3-):
How does that change our water quality?
Bicarb adds inorganic carbon
The bicarbonate ion carries a carbon atom (the C in HCO3-). That adds one unit of DIC, which changes our water quality.
Specifically, the added DIC moves us one unit to the right on the Water Quality Map, parallel to the x-axis.
Bicarb also adds alkalinity
The bicarbonate ion also carries a negative charge (HCO3-). That adds one unit of alkalinity, which also changes our water quality.
The added alkalinity moves us one unit up, parallel to the y-axis.
[OPTIONAL] What about the Na+?
Let's see what adding bicarb looks like on the Water Quality Map.
In the next map, we start at , our initial waypoint.
We then take one step to the right, parallel to the DIC axis. That represents adding one unit of DIC (1 mmol/kg).
We then take one step up, parallel to the alkalinity axis. That represents adding one unit of alkalinity (1 meq/kg).
And we end up at a point on the blue bicarbonate vector.
And if we added, say, 2.68 units of bicarb, we'd then take 2.68 steps to the right and 2.68 steps up.
That would put us at a different point on the same bicarb vector.
So, when we add bicarb, we plot it as a vector with a slope of 1 (i.e., a 45° angle) directed toward the upper right of the map.
We now have everything we need to visualize the effect of adding NaHCO3 on the pH of our culture water.
This allows us to do an end-run around all of the complicated chemistry and math by simply comparing the slope of the bicarb vector with the slopes of the local pH lines.
Three cases are illustrated next, each with a screenshot from the Water Quality Map.
(A link at the bottom of this post takes you to an interactive version of the Water Quality Map so that you can play with it yourself.)
The Familiar Case: Bicarb RAISES pH
Our starting point is pH 6.0, the red pin in the next map.
This is lower than the typical pH of most commercial culture systems, but we'll use it here to emphasize our point.
The screenshot below shows the result of adding 13 kg (28.7 lbs) of bicarb to our 100-m3 system.
That added bicarb raises pH from 6.0 to 6.25.
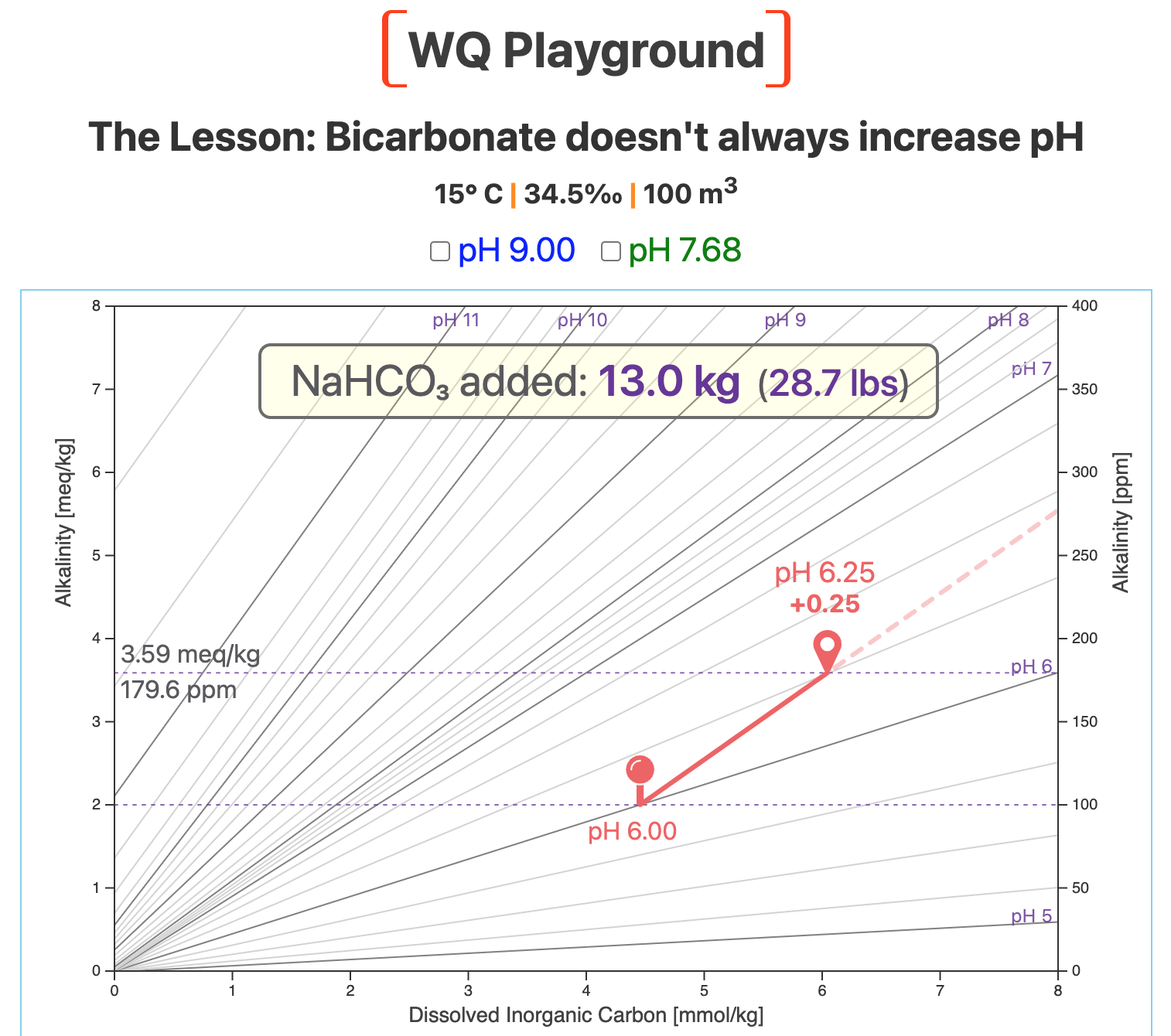
Now compare the slope of the bicarb vector with the slopes of the nearby pH lines.
The bicarb slope is steeper than the slopes of the local pH lines.
Because the slope of the bicarb vector is steeper in this region of the map, it cuts across lines of higher and higher pH.
The result is what most aquaculturists expect: Adding sodium bicarbonate increases pH — in this region of the map.
So, we started with a relatively low initial pH, and adding bicarb raised it — but not by very much.
That's a lot of bicarb to add for a pretty small pH change. (Not very economical over a crop cycle, eh?)
Note that alkalinity increased by about 1.6 meq/kg (~80 ppm).
The next two cases demonstrate that sodium bicarbonate doesn't always increase pH.
The Suprising Case: Bicarb LOWERS pH
This is a new one for many people.
The blue pin marks our new starting point: pH 9.0.
That's much higher than we'd ever have in fish or shrimp culture. At that pH, even low levels of Total Ammonia would mean high levels of toxic Un-Ionized Ammonia.
You might find that pH (or higher) in high-density microalgae tanks, but we again are using it here to emphasize our point.
The next screenshot shows the result of adding 13 kg (28.7 lbs) of bicarb to our 100-m3 system.
That LOWERS pH from 9.0 to 8.65, a decrease of 0.35 pH units.
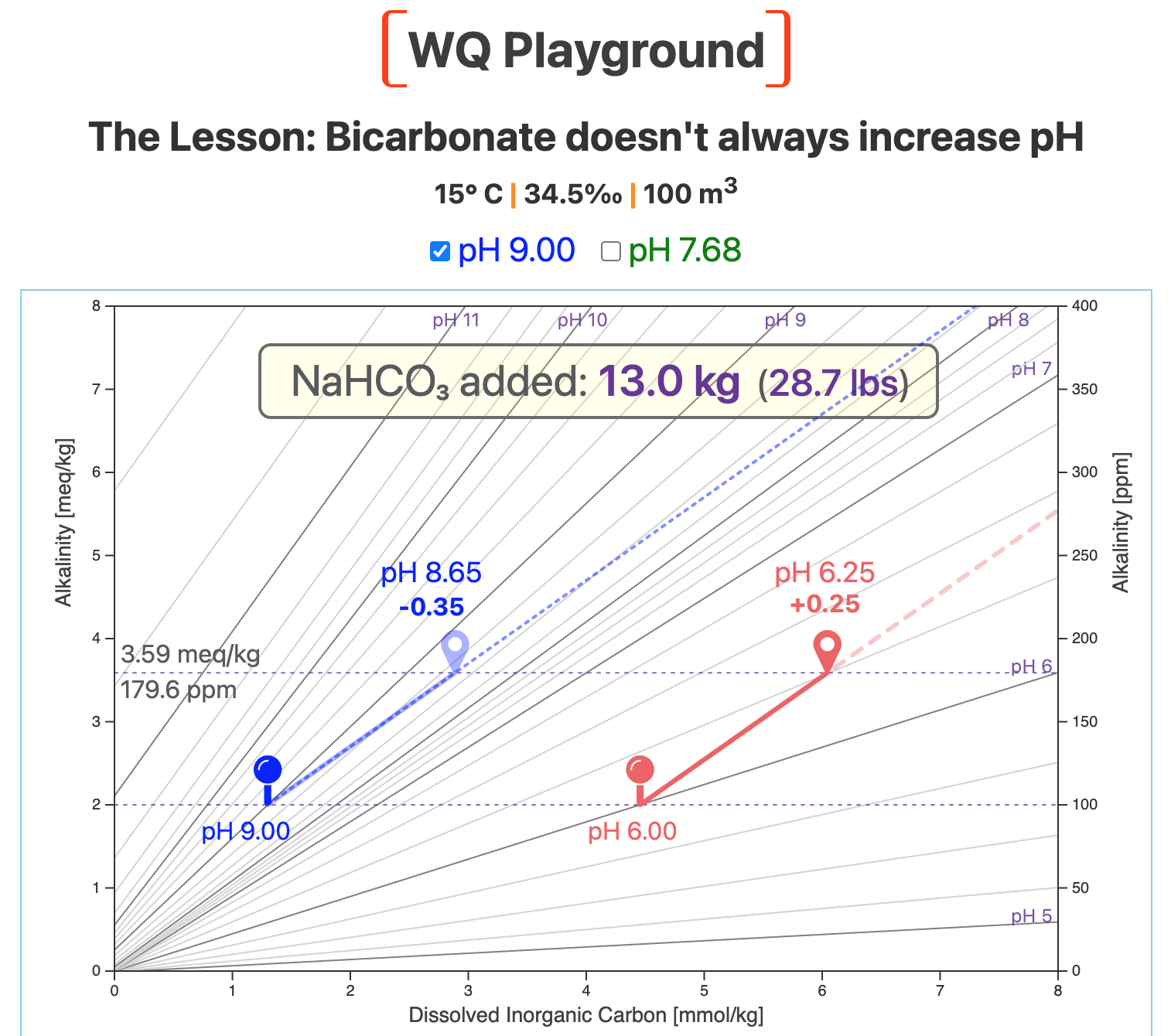
Compare the slope of the blue bicarb vector with the slopes of the nearby pH lines.
The bicarb slope is less steep than those of the local pH lines.
Because the bicarb slope is less steep in this region of the map, it cuts across lines of lower and lower pH.
This result surprises most aquaculturists: Adding sodium bicarbonate decreases pH — in this region of the map.
Note that alkalinity still increased by ~1.6 meq/kg (~80 ppm).
The last case is the most relevant and, understandably, a source of confusion for many aquaculturists.
The Confusing Case: Is my pH meter broken?!
We've seen that...
-
...when pH is low, adding bicarbonate raises pH
-
...when pH is high, adding bicarbonate lowers pH
As you may have guessed, there's a pH between those low and high values where adding bicarbonate has no effect on pH at all.
We'll demonstrate that here by starting at pH 7.68, which is not an unusual pH for many aquaculture, aquaponics, home aquarium, and residential swimming pool systems.
The next screenshot shows the result of adding almost 25 kg (55 lbs) of bicarb to our 100-m3 system, initially at pH 7.68 ().
Adding that significant amount of NaHCO3
has no effect on the pH of our culture water, which stays at pH 7.68.
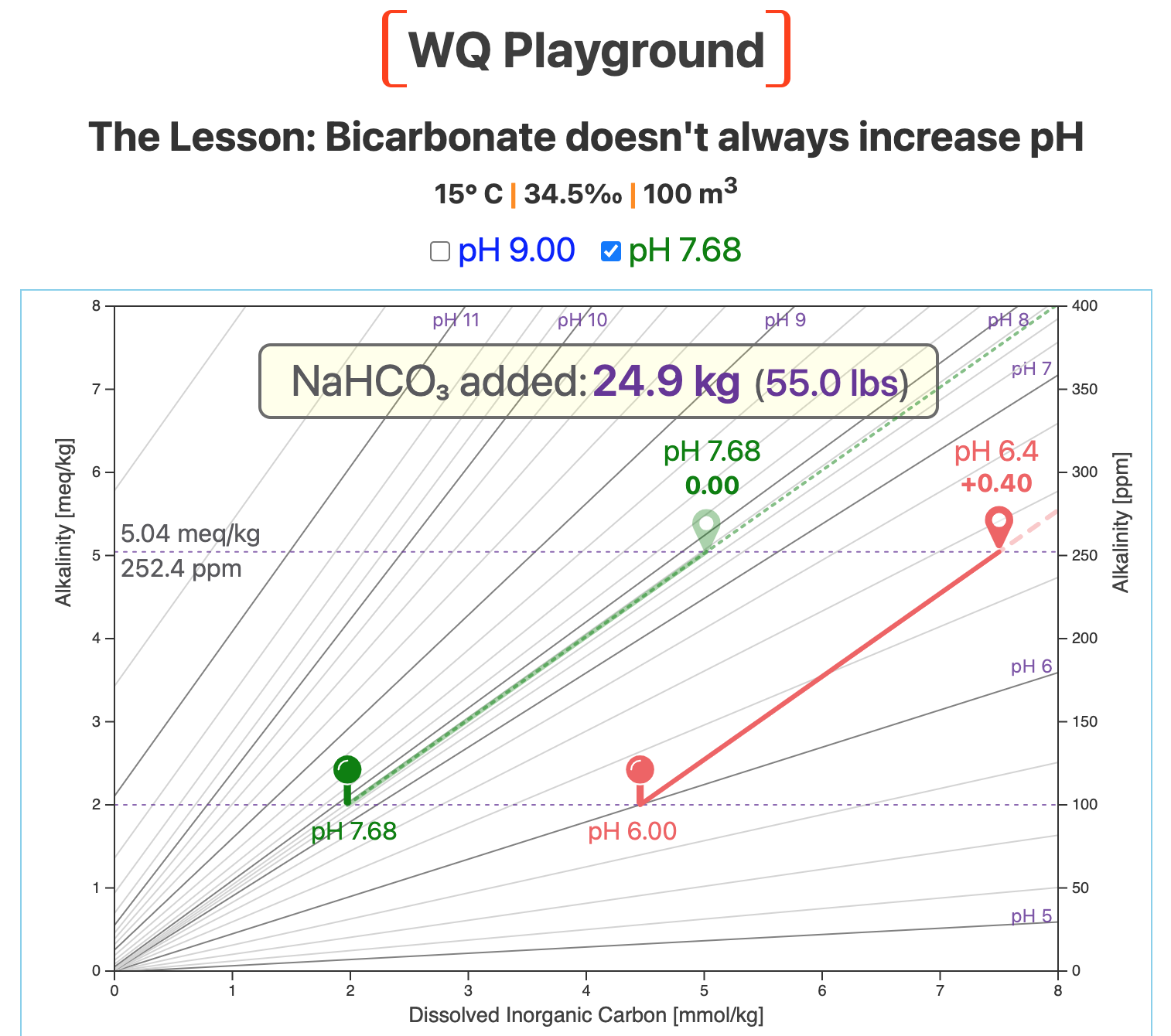
We chose pH 7.68 for this example on purpose:
For our temperature and salinity, the slope of the pH 7.68 line very closely matches the slope of the green bicarb vector.
It would be very rare, of course, for your pH to be exactly at that special value (at your water temperature and salinity).
But it's very likely that your pH would be within the range in which adding NaHCO3 has very little effect on pH.
In that case, you'd find — as others have — that adding even a massive amount of sodium bicarbonate has little effect on pH.
As a result, you'd be wasting money on bags of bicarb that don't help you manage your culture environment.
You'd also be pushing your alkalinity to very high levels.
In our example, alkalinity increased to ~5 meq/kg (~252 ppm). That's more than twice the alkalinity of standard seawater.
That would affect not only your bank account, but also your water quality. (Excess alkalinity is a topic for another post.)
Bicarb wrap-up
Sodium bicarbonate...
-
...alone is not an economical way to manage pH
-
...always increases alkalinity, which can lead to high-alk problems
-
...adds sodium, which upsets the sodium:potassium (Na:K) ratio
For precise water-quality control, sodium bicarbonate must be used with at least one other reagent.
Which other reagent(s)?
There are several options for pH-alkalinity adjustment, including sodium carbonate (Na2CO3, soda ash), sodium hydroxide (NaOH, caustic soda), calcium hydroxide (Ca(OH)2, slaked lime), and others.
We'll also mention potassium chloride (KCl, muriate of potash) for tweaking the Na:K ratio.
That is, adding sodium increases the Na:K ratio. When that ratio is too high, you have to add enough potassium to reduce it to its normal seawater (or freshwater) value.
The way that each of those reagents influences the state of your culture water will be discussed in other posts.